
2024年10月29日,礼来Eli Lilly宣布Donanemab(商品名:Kisunla)TRAILBLAZER-ALZ 6研究3b期临床试验取得积极结果。
发布时间:2024-10-29浏览数:1k+

2024年7月2日,礼来公司宣布FDA批准Donanemab(商品名Kisunla™)350 mg/20 mL,每月一次静脉输注用于治疗处于轻度痴呆阶段,伴有轻度认知障碍(MCI),并经淀粉样蛋白病理学确诊的成人早期症状性…
发布时间:2024-07-02浏览数:1k+
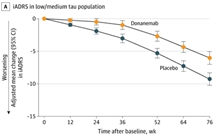
2023年7月17日Eli Lilly公布了抗β淀粉样蛋白(Aβ)单克隆抗体Donanemab的3期临床试验TRAILBLAZER-ALZ 2试验结果。试验显示 Donanemab能够有效改善Aβ斑块检测阳性阿尔茨海默症早期症状患者的认…
发布时间:2023-07-17浏览数:1k+

Among participants with early symptomatic Alzheimer disease and amyloid and tau pathology, donanemab significantly slowed clinical progression at 76 weeks in those with low/medium t…
发布时间:2023-07-17浏览数:1k+
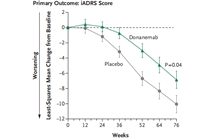
In patients with early Alzheimer's disease, donanemab resulted in a better composite score for cognition and for the ability to perform activities of daily living than placebo at 76…
发布时间:2021-05-06浏览数:905
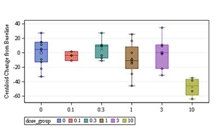
Intravenous donanemab 10 mg/kg can reduce amyloid deposits in AD despite having a shorter than expected half-life.
发布时间:2021-02-14浏览数:1k+
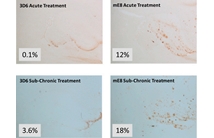
These studies have profound implications for the development of therapeutic Aβ antibodies for Alzheimer's disease.
发布时间:2012-01-06浏览数:1k+
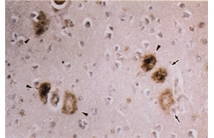
A beta N3(pE) production and retention may play an early and critical role in senile plaque formation.
发布时间:1995-02-01浏览数:915
 改变滴定方案礼来降低其阿尔茨海默症抗体药物脑水肿发生率
改变滴定方案礼来降低其阿尔茨海默症抗体药物脑水肿发生率横切线®为注册商标
Copyright 2020 横切线®药研苑 备案号:粤ICP备18041379号-3