
2024年5月14日卫材Eisai宣布已开始滚动向FDA提交预充式自动注射仑卡奈单抗的生物制品上市许可申请(BLA)。
发布时间:2024-05-14浏览数:1k+

2024年05月08日,Acumen Pharmaceuticals宣布2期临床试验ALTITUDE-AD首位阿尔茨海默病(AD)患者已接受Sabirnetug(ACU193)给药。
发布时间:2024-05-08浏览数:697
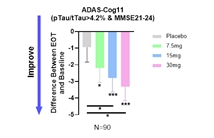
2024年4月29日 Annovis Bio发布Buntanetap治疗轻至中度阿尔茨海默症(AD)2/3期临床试验的试验数据。
发布时间:2024-04-29浏览数:1k+

2024年3月11日,Allyx Therapeutics宣布在1b期多次递增剂量临床试验中,ALX-001每日口服2次,所有测试剂量,对认知功能正常老年受试者都表现出良好的安全性。
发布时间:2024-03-11浏览数:707

2024年2月14日Annovis Bio在线宣布Buntanetap治疗轻至中度阿尔茨海默症的2/3期临床试验,最后一名患者完成最后一次访视。
发布时间:2024-02-14浏览数:698

2024年1月31日渤健(Biogen)宣布调整抗阿尔茨海默症药物开发管线,停止抗β淀粉样蛋白(Aβ)单克隆抗体Aduhelm(Aducanumab)的开发,并终止 Aducanumab的临床试验ENVISION。
发布时间:2024-01-31浏览数:682

2024年1月9日,中国上海——卫材(中国)药业有限公司宣布抗阿尔茨海默症药物乐意保(Leqembi)®(仑卡奈单抗Lecanemab)获国家药品监督管理局(NMPA)批准正式进入中国。
发布时间:2024-01-09浏览数:1k+
AL001是由美国南佛罗里达大学(University of South Florida)的Douglas Ronald Shytle和Jun Tan合作开发的用于治疗阿尔茨海默症(AD)的水杨酸锂L-脯氨酸共晶(LISPRO),已完成治疗AD的2a期临床…
发布时间:2023-10-02浏览数:891
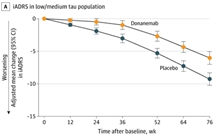
2023年7月17日Eli Lilly公布了抗β淀粉样蛋白(Aβ)单克隆抗体Donanemab的3期临床试验TRAILBLAZER-ALZ 2试验结果。试验显示 Donanemab能够有效改善Aβ斑块检测阳性阿尔茨海默症早期症状患者的认…
发布时间:2023-07-17浏览数:1k+

Among participants with early symptomatic Alzheimer disease and amyloid and tau pathology, donanemab significantly slowed clinical progression at 76 weeks in those with low/medium t…
发布时间:2023-07-17浏览数:1k+
横切线®为注册商标
Copyright 2020 横切线®药研苑 备案号:粤ICP备18041379号-3